A pill taken once a week. A shot administered at home once a month. Even a jab given at a clinic every six months.
In the next five to 10 years, these options may be available to prevent or treat H.I.V. Instead of drugs that must be taken daily, scientists are closing in on longer-acting alternatives — perhaps even a future in which H.I.V. may require attention just twice a year, inconceivable in the darkest decades of the epidemic.
“This period is the next wave of innovation, newer products meeting the needs of people, particularly in prevention, in ways that we didn’t ever have before,” said Mitchell Warren, executive director of the H.I.V. prevention organization AVAC.
Long-acting therapies may obviate the need to remember to take a daily pill to prevent or treat H.I.V. And for some patients, the new drugs may ease the stigma of the disease, itself an obstacle to treatment.
“To not have to remember that every morning is earth-changing for them,” said Dr. Rachel Bender Ignacio, an infectious diseases physician and researcher at the Fred Hutch Cancer Center in Seattle. “That stigma, that internalized stigma of taking that pill every morning, is what prevents them from taking it.”
Long-acting drugs are likely to be an even greater boon in populations that have long been hard to reach: patients who have spotty access to health services, or who have trouble taking daily pills because they have unstable housing or transportation, are struggling with substance use, are mentally ill or face discrimination and stigma.
In 2022, nearly 30 years after the advent of combination antiretroviral therapy, more than nine million of the 39 million people living with H.I.V. worldwide were not receiving treatment. About 630,000 died from AIDS-related illnesses that year.
Even in the United States, about one-third of those diagnosed with H.I.V. are not keeping the virus in check. “We still haven’t addressed these sort of underlying issues around access,” said Gregg Gonsalves, a longtime H.I.V. activist and an epidemiologist at the Yale School of Public Health.
“We can be elated about the science and the clinical implications” of long-lasting drugs, he added. “But for many people, it’s going to be a distant dream.”
One barometer of the excitement about long-acting regimens was their prominence at the Conference on Retroviruses and Opportunistic Infections in Denver in March. The annual meeting has served as the backdrop to many H.I.V. milestones, including the electric moment in 1996 when researchers showed that a combination of drugs could suppress the virus.
Dozens of studies of long-acting regimens were presented at the conference this year. (While most such drugs are tantalizingly close for H.I.V. prevention and treatment, similar options for tuberculosis, hepatitis B and hepatitis C are not far behind.)
One long-acting treatment — Cabenuva, two shots given every other month — has been available for nearly three years. It costs more than $39,000 annually in the United States, although few patients pay that price. Even with a steep discount, however, the treatment is out of reach for many patients in low-income countries.
Still, many researchers at the conference were excited about the results from one study showing that Cabenuva was more effective than daily pills at controlling H.I.V. even in groups that typically have trouble adhering to treatment.
“When you think about how hard it is for some folks, giving them new tools that might be able to get them to be suppressed is a big deal,” said Dr. Kimberly Smith, who leads research and development at ViiV Healthcare, which makes one of the component drugs in Cabenuva.
Long-acting drugs might be useful even for children living with H.I.V. Worldwide, only about half of children diagnosed with H.I.V. are receiving treatment.
That’s in part because of the lack of drug versions made for children, Dr. Charles Flexner, an H.I.V. expert at Johns Hopkins University, said in a presentation at the Denver conference.
“With long-acting formulations, that will no longer be the case,” Dr. Flexner said. “Children will be able to use the same formulation as adults, just at a different dose.”
Most long-acting shots contain nanocrystals of drug suspended in liquid. While oral pills must pass through the stomach and the intestinal tract before they enter the circulation, so-called depot shots deliver the drugs directly into the bloodstream. But they are released extremely slowly, over the course of weeks or months.
Some depot antipsychotics are given every two to eight weeks, and the contraceptive Depo-Provera is administered once every three months. Cabenuva — a combination of cabotegravir, made by Viiv Healthcare (majority owned by GSK), and Janssen’s rilpivirine — is injected into gluteal muscles every two months to treat H.I.V.
Cabotegravir given under the skin of the stomach produced more bruising and rashes than in the buttocks, and some people developed nodules that lingered for weeks or even months. But with gluteal injections, “there’s nothing that you see,” Dr. Smith said. “You feel pain for a couple of days and then you go on with your life.”
Viiv is trying to develop a version of cabotegravir to be given every four months and, ultimately, one every six months. The company aims to bring the four-month version to market for preventing H.I.V. in 2026, and for treatment in 2027.
But injecting drugs into muscle is challenging for people who have significant body fat or who have silicone implants in the buttocks, as some trans women do. Some newer shots under development are administered under the skin, circumventing the problem.
Gilead’s lenacapavir can be given as a subcutaneous injection in the stomach once every six months, but it is so far approved only for people with H.I.V. who are resistant to other drugs. The drug is in multiple late-stage trials as a long-acting H.I.V. preventative in various groups, including cisgender women.
Lenacapavir is also being tested as a treatment in the form of a once-weekly pill in combination with another drug, islatravir, made by Merck. Having multiple long-acting treatments is ideal, “so people can really make the choice among the options that are going to work best for them,” said Dr. Jared Baeten, a vice president at Gilead.
Santos Rodriguez, 28, was diagnosed with H.I.V. in 2016 and has taken a daily pill ever since to suppress the virus. Mr. Rodriguez, who works on artificial intelligence at Mayo Clinic in Florida, said having to take only one pill a week would be “definitely groundbreaking for me and my adherence.”
He said he was put off by the clinic visits every two months required for Cabenuva shots, and by reports that injections in the buttocks are painful. A shot every four months or every six months would be much more attractive, he added.
To make it truly accessible for everyone, including those who may live far from a health care center, researchers must also come up with a long-acting injection that can be self-administered, some experts noted.
One team is developing exactly that and, with backing from the global health initiative Unitaid, planning to make it available in low- and middle-income nations.
“The really exciting thing about this is that the way that it’s being developed, it ideally will bypass the trickle-down effect to get into the people who need it most,” said Dr. Bender Ignacio, referring to the tendency of rich countries to gain access to new therapies first. She is leading the study.
The product uses a lipid base to suspend three H.I.V. drugs, two water-soluble and one fat-soluble. Unlike depot shots, which release drugs slowly, this so-called nanolozenge is taken up by the immune cells and lymph nodes immediately after it is delivered under the skin of the stomach.
The shots can carry smaller doses of drugs because of this efficiency, and they can also easily be adapted for children and adolescents, Dr. Bender Ignacio said. A single injection maintains levels of the three drugs in the body for more than a month, replacing 150 pills.
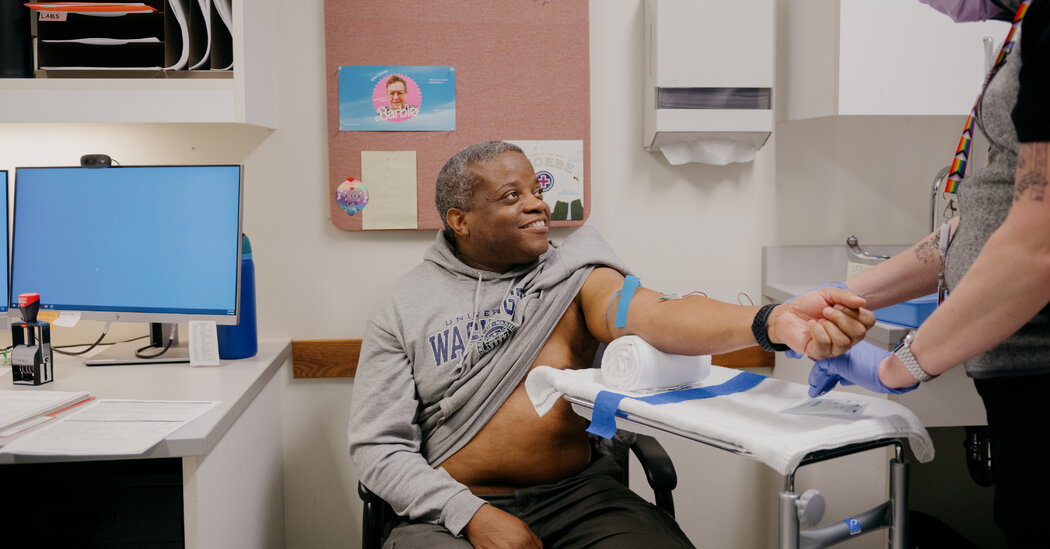
So far, the self-administered long-acting shot has been tested in just 11 people, including Kenneth Davis, 58, a resident of Auburn, Wash. Mr. Davis, who lost two family members to AIDS, likened the jab to a bee sting — fleeting and less painful than the Covid vaccines.
Because the component drugs have each been independently approved, Dr. Bender Ignacio estimated the shots could be available to treat H.I.V. in less than five years.
Many of the products, including those in Dr. Bender Ignacio’s study, can be adjusted to prevent H.I.V. There are currently only three options for that: two types of daily pills, and Viiv’s cabotegravir, which is injected into the buttocks once every two months.
“It’s been prevention where we have been lagging the greatest in the AIDS response over the last decade,” Mr. Warren, of AVAC, said.
One study presented at the Denver conference showed that when people were offered a choice of prevention methods, more of them chose long-acting cabotegravir. But the percentage opting for daily pills also rose.
“The fact that we saw protection go up with a range of methods — that to me is the most important thing,” Mr. Warren said. The study, he added, “really shows that there is now evidence behind choice, not just advocacy.”